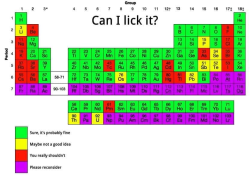
Superacid vs. Tangerine
The classical definition of a superacid is any acid with an acidity greater than that of 100% pure sulfuric acid (H2SO4). In highly concentrated solutions, the Henderson-Hasselbalch equation used to define the pH scale breaks down due to large variations in the activity coefficients. In order to measure the acidity of very concentrated solutions, a similar metric known as the Hammett acidity function (H0) was introduced. Using this scale, 100% sulfuric acid has an H0=-12.
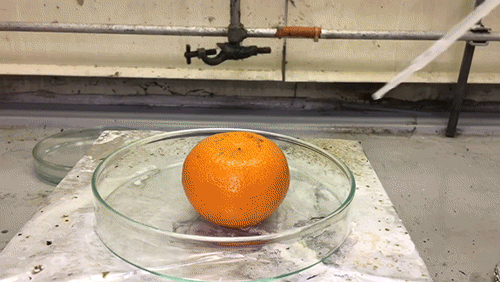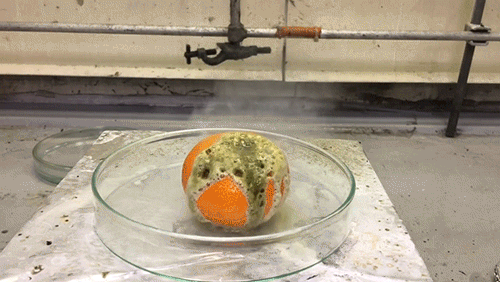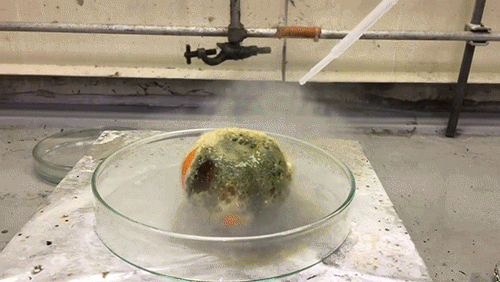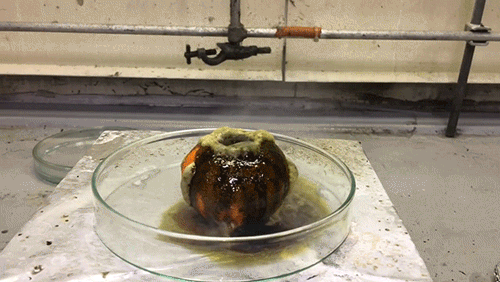
The acid used in this GIF is chlorosulfuric acid (HSO3Cl), which has an H0=-12.78, therefore making it a superacid. It reacts violently with the water in the tangerine skin to yield sulfuric acid and hydrogen chloride (HCl) gas. The sulfuric acid then reacts with the sucrose of the tangerine via an intense exothermic dehydration reaction to produce carbon in its black graphite form.